Photoinduced proton transfer inside an engineered green fluorescent protein: a stepwise–concerted-hybrid reaction†
Abstract
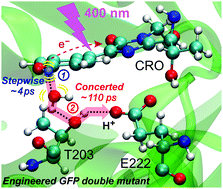
Photoactivated proton transfer (PT) wire is responsible for the glow of green fluorescent protein (GFP), which is crucial for bioimaging and biomedicine. In this work, a new GFP-S65T/S205V double mutant is developed from wild-type GFP in which the PT wire is significantly modified. We implement femtosecond transient absorption (fs-TA) and femtosecond stimulated Raman spectroscopy (FSRS) to delineate the PT process in action. The excited state proton transfer proceeds on the ∼110 ps timescale, which infers that the distance of one key link (water to T203) in the PT wire of GFP-S205V is shortened by the extra S65T mutation. The rise of an imidazolinone ring deformation mode at ∼871 cm−1 in FSRS further suggests that this PT reaction is in a concerted manner. A ∼4 ps component prior to large-scale proton dissociation through the PT wire is also retrieved, indicative of some small-scale proton motions and heavy-atom rearrangement in the vicinity of the chromophore. Our work provides deep insights into the novel hybrid PT mechanism in engineered GFP and demonstrates the power of tunable FSRS methodology in tracking ultrafast photoreactions with the desirable structural specificity in physiological environments.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...