From ascorbic acid to furan derivatives: the gas phase acid catalyzed degradation of vitamin C†
Abstract
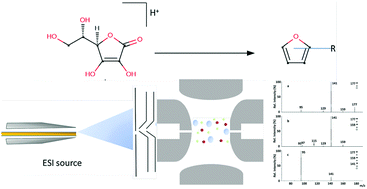
Furan derivatives, potentially carcinogenic to humans, can be formed, in addition to carbohydrates and other sources, from the degradation of ascorbic acid (AA). At present, the mechanisms involved in the ascorbic acid degradation are not yet fully understood. In this study, we reported a gas-phase investigation, performed using Triple Quadrupole (TQ/MS) and Ion Trap Mass Spectrometry (QIT/MS) together with quantum mechanical calculations at the B3LYP/6-31+G(d,p) level of theory, on the non-oxidative degradation mechanism of L-ascorbic acid (AA) to furan derivatives. Gaseous protonated ascorbic acid ions, the AAH+ ionic reagents, were generated by Electrospray Ionization (ESI) of an aqueous AA solution added with a mild protonation reagent. The Collisionally Induced Dissociation (CID) mass spectra of the AAH+ ions displayed gaseous fragment ions corresponding to the degradation intermediates and reaction products, which were structurally characterized and identified. Precise mechanistic insights were achieved by using L-[1-13C]-AA. On the basis of experimental and computational results, the gas phase non-oxidative acid catalyzed degradation mechanism of ascorbic acid is proposed, a benchmark point for the comprehension of the mechanism in the condensed phase.


 Please wait while we load your content...
Please wait while we load your content...