Thermodynamics of the interactions of positively charged cellulose nanocrystals with molecules bearing different amounts of carboxylate anions†
Abstract
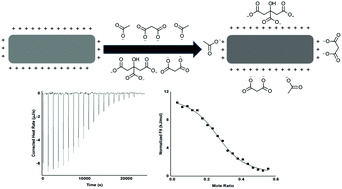
Negatively charged ions readily interact with the surface of positively charged pyridinium-grafted cellulose nanocrystals. In this work we investigated the thermodynamics of these interactions using isothermal titration calorimetry. We investigated the effect of the ionic charge, using carboxylate salts with different valence (1–4), and compared it with sodium sulfate as another delocalized ionic charge. Experiments performed using cellulose nanocrystals with three different degrees of substitution of pyridinium grafts showed that the number of ions adsorbed onto the surface of cellulose nanocrystals is directly linked to the number of grafted cationic functionalities. The adsorption of anions onto pyridinium-grafted nanocrystals was further found to be endothermic and driven by an increase in entropy upon adsorption of the anions due to the release of surface bound water. The association constant increased with the net charge of the anions from a low value for monovalent sodium acetate. Both entropy and enthalpy increased linearly with the net charge of the anions, demonstrating that a stronger depolarization occurred when anions of higher net charge were adsorbed. The values of the stoichiometric number determined were lower than the values relative to processes involving ions in solution, suggesting a more complex mode of interaction, involving hydrogen-bonding and bridge formation. However, the number of adsorbed ions was directly correlated with the amount of cationic surface grafts. This makes it possible to control the amount of surface interactions directly with the degree of substitution on the nanocrystal surfaces. Comparison with zeta potential measurements showed that zeta-potential measurements can be used as a direct method to determine the stoichiometry of binding between the positive surface grafts and the carboxylate salts.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...