Lithium doped tubular structure in LiB20 and LiB20−: a viable global minimum†
Abstract
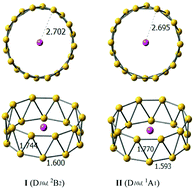
We present a strategy by which the stability of tubular boron clusters can be significantly enhanced by doping the B20 cluster with a lithium atom. High-level quantum chemical calculations showed that the lowest energy structures of LiB20 and LiB20− are tubular structures with D10d symmetry, in which the lithium atom is located at the center of the tubular structure. Chemical bonding analysis revealed that the high-symmetry tubular boron clusters are characterized as charge transfer complexes (Li+B20− and Li+B202−), resulting in double aromaticity with delocalized π + σ bonding and strong electrostatic interactions between cationic Li+ and tubular boron motifs with twenty Li–B interactions. The unique bonding pattern of the LiB20 and LiB20− species provides a key driving force to stabilize tubular structures over quasi-planar structures, suggesting that electrostatic interactions resulting from alkali metals might unveil a new clue to the structural evolution of boron clusters.


 Please wait while we load your content...
Please wait while we load your content...