Steered molecular dynamics simulations reveal the role of Ca2+ in regulating mechanostability of cellulose-binding proteins†
Abstract
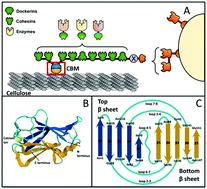
The conversion of cellulosic biomass into biofuels requires degradation of the biomass into fermentable sugars. The most efficient natural cellulase system for carrying out this conversion is an extracellular multi-enzymatic complex named the cellulosome. In addition to temperature and pH stability, mechanical stability is important for functioning of cellulosome domains, and experimental techniques such as Single Molecule Force Spectroscopy (SMFS) have been used to measure the mechanical strength of several cellulosomal proteins. Molecular dynamics computer simulations provide complementary atomic-resolution quantitative maps of domain mechanical stability for identification of experimental leads for protein stabilization. In this study, we used multi-scale steered molecular dynamics computer simulations, benchmarked against new SMFS measurements, to measure the intermolecular contacts that confer high mechanical stability to a family 3 Carbohydrate Binding Module protein (CBM3) derived from the archetypal Clostridium thermocellum cellulosome. Our data predicts that electrostatic interactions in the calcium binding pocket modulate the mechanostability of the cellulose-binding module, which provides an additional design rule for the rational re-engineering of designer cellulosomes for biotechnology. Our data offers new molecular insights into the origins of mechanostability in cellulose binding domains and gives leads for synthesis of more robust cellulose-binding protein modules. On the other hand, simulations predict that insertion of a flexible strand can promote alternative unfolding pathways and dramatically reduce the mechanostability of the carbohydrate binding module, which gives routes to rational design of tailormade fingerprint complexes for force spectroscopy experiments.


 Please wait while we load your content...
Please wait while we load your content...