Stereochemistry-dependent structure of hydrogen-bonded protonated dimers: the case of 1-amino-2-indanol†
Abstract
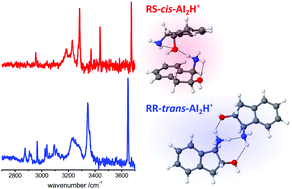
To understand the role of chirality in shaping biological supramolecular systems it is instructive to visualize the subtle effects of stereochemistry on the structure of model aggregates at the molecular level. Here, we apply conformer-specific IR-UV double-resonance laser spectroscopy in a cold ion trap to derive a detailed description of the protonated homodimers of (1R,2S)-cis- and (1R,2R)-trans-1-amino-2-indanol (c-AI2H+, t-AI2H+). Although the protonated monomers (c-AIH+, t-AIH+) only differ by the chirality of one carbon atom, their conformations are clearly distinct. c-AIH+ has an intramolecular NH+⋯O hydrogen bond (H-bond), while t-AIH+ lacks such an interaction. This has crucial consequences on the geometry and stability of the corresponding c-AI2H+ and t-AI2H+ dimers. While there is a competition between intra- and intermolecular H-bonds in c-AI2H+, the formation of t-AI2H+ does not require deformation of the monomers. This difference results in higher binding energies of t-AI2H+ compared to c-AI2H+. To optimize the H-bond network, the two dimers do not necessarily involve the corresponding most stable monomers. c-AI2H+ and t-AI2H+ differ in their UV photodissociation mass spectra and in their electronic spectra, which suggests different geometries also in the excited state.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...