Effects of nanostructuring on the bond strength and disorder in V2O5 cathode material for rechargeable ion-batteries
Abstract
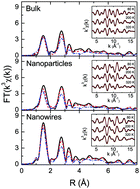
We have investigated the nanostructuring effects on the local structure of V2O5 cathode material by means of temperature dependent V K-edge X-ray absorption fine structure measurements. We have found that the nanostructuring largely affects V–O and V–V bond characteristics with a general softening of the local V–O and V–V bonds. The obtained bond strengths correlate with the specific capacity shown by the different systems, with higher capacity corresponding to softer atomic pairs. The present study suggests the key role of local atomic displacements in the diffusion and storage of ions in cathodes for batteries, providing important information for designing new functional materials.


 Please wait while we load your content...
Please wait while we load your content...