Clathrate ice sL: a new crystalline phase of ice with ultralow density predicted by first-principles phase diagram computations†
Abstract
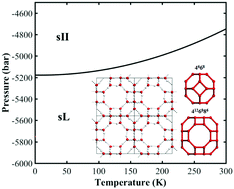
In contrast to the rich knowledge of water and 17 experimentally confirmed crystalline phases of solid water under positive pressures, water under negative pressure has been poorly explored. In this study, a new crystalline phase of ice with ultralow density (0.6 g cm−3), named “clathrate ice sL”, is constructed by nano water cage clusters, and it is predicted to be stable under a lower negative pressure than the experimentally confirmed sII phase by first-principles phase diagram computations, thereby extending the phase diagram of water to negative pressure regions below −5170 bar at 0 K and below −4761 bar at 300 K. In addition, according to our theoretical prediction, the optimal hydrogen storage mass density in the new clathrate ice sL is 7.7 wt% (larger than the 2017 DOE target of 5.5 wt%), which would set a new record of hydrogen storage capacity in clathrate hydrates. The finding of clathrate ice sL not only proposes a new type of crystalline ice under negative pressure but also explores the potential applications of the ultralow density ice phases while extending the water phase diagram and enriching the knowledge of people about water.


 Please wait while we load your content...
Please wait while we load your content...