Optical spectroscopy of isolated flavins: photodissociation of protonated lumichrome†
Abstract
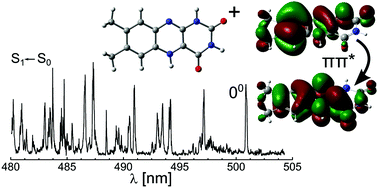
The optical properties of flavins strongly depend on the charge and oxidation states as well as the environment. Herein, the electronic spectrum of cold protonated lumichrome, the smallest flavin molecule, is recorded by means of photodissociation in the visible range (VISPD) in a cryogenic ion trap tandem mass spectrometer coupled to an electrospray ionization source. The vibronic spectrum is assigned to the S1 ← S0 (ππ*) transition of the most stable N5-protonated isomer by comparison with quantum chemical calculations at the PBE0/cc-pVDZ level in combination with multidimensional Franck–Condon simulations. Analysis of the geometric and electronic structures of neutral and protonated lumichrome explains the large red shift of the band origin upon protonation (ΔS1 ∼ −6000 cm−1), which corresponds to the increase in proton affinity upon S1 excitation as a result of charge transfer. N5 protonation greatly modifies the structure of the central pyrazine ring of the chromophore. The orbitals involved in S1 ← S0 excitation include an important fraction of the probability at the central ring and they are, hence, largely influenced by the positive charge of the attached proton. The rich vibronic spectrum indicates the large geometry change upon S1 excitation. This combined experimental and computational approach is shown to be suitable to determine the optical properties of flavins as a function of oxidation, protonation, metalation, and microsolvation state.


 Please wait while we load your content...
Please wait while we load your content...