Unimolecular decomposition of formamide via direct chemical dynamics simulations†
Abstract
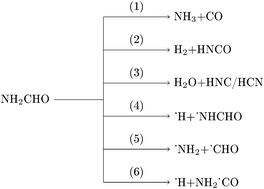
Formamide (NH2CHO), being the simplest organic molecule containing an amide functional group, serves as a prototype to study protein and peptide chemistry. Formamide has been found in Comets and interstellar media and its decomposition results in smaller molecules such as NH3, CO, HCN, HNCO, etc. These smaller molecules are considered to have been potential precursors for the formation of complex biological molecules, such as nucleic acids and nucleobases, in the early Earth. Several experimental and theoretical investigations of formamide decomposition have been reported in the literature. In the present work, unimolecular decomposition of formamide in the electronic ground state was investigated by classical direct chemical dynamics simulations. The calculations were performed at three different energies using the density functional B3LYP/aug-cc-pVDZ level of electronic structure theory. The major dissociation products observed were NH3, CO, H2, HNCO, H2O, HCN, and HNC along with products of a few minor dissociation channels. Reactivity, atomic level mechanisms, and product branching ratios were investigated as a function of total energy.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...