Valence shell threshold photoelectron spectroscopy of C3Hx (x = 0–3)†
Abstract
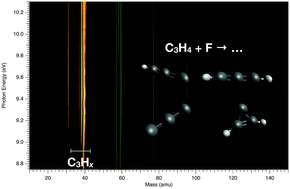
We present the photoelectron spectra of C3Hx (x = 0–3) formed in a microwave discharge flow-tube reactor by consecutive H abstractions from C3H4 (C3Hx + F → C3Hx−1 + HF (x = 1–4)), but also from F + CH4 schemes by secondary reactions. The spectra were obtained combining tunable VUV synchrotron radiation with double imaging electron/ion coincidence techniques, yielding mass-selected threshold photoelectron spectra. The obtained results complement not only existing ones, but for the first time the photoelectron spectra of C3, cyclic and linear C3H (c,l-C3H) as well as of the excited states of C3H3 are reported. In the case of c-C3H, l,t-C3H2 and C3H3, Franck–Condon simulations have been performed in order to assign the vibrational structure. The adiabatic ionization energies of these radicals are reported and compared to ab initio calculated values as well as to theoretical values using known enthalpies of formation.


 Please wait while we load your content...
Please wait while we load your content...