Understanding the hydrogen transfer mechanism for the biodegradation of 2,4,6-trinitrotoluene catalyzed by pentaerythritol tetranitrate reductase: molecular dynamics simulations†
Abstract
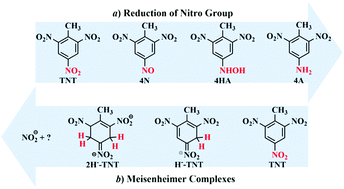
The explosive 2,4,6-trinitrotoluene (TNT) is a highly toxic pollutant. Biodegradation is inevitably one of the most cost-effective and enviromentally friendly means of removing TNT pollution. However, the aromatic derivatives from the reduction of nitro groups by several classic enzymes are still toxic. Besides the reduction of nitro groups, pentaerythritol tetranitrate reductase (PETNR) offers a potential route to ring fission and complete degradation of TNT through the pathway of the Meisenheimer complex. This work is devoted to deeply understand the essence of the Meisenheimer pathway and mainly focus on the crucial hydrogen-transfer reaction by means of molecular dynamics (MD) simulations. We obtain three valuable findings. Firstly, the parallel π–π stacking between TNT and the flavin mononucleotide (FMN) cofactor is a precondition. The key residue controlling this conformation is His181. Although His184 does not interact with TNT, the mutation from His184 to Asn184 would abolish the π–π structure. Secondly, the data of the empirical valence bond (EVB) show that the Meisenheimer pathway is predominant because its activation barrier is 6.7 kcal mol−1 far less than that of nitro reduction (26.6 kcal mol−1). Finally, based on the results of thermodynamic integration (TI), the type of transferred hydrogen is also ensured, that is, the H anion (H−) for the Meisenheimer complex and the H radical (H˙) for nitro reduction. Our findings provide an exhaustive understanding for the first hydrogen transfer reaction that has a decisive effect on two competing pathways, and help in searching for and designing new enzymes that can effectively degrade TNT.


 Please wait while we load your content...
Please wait while we load your content...