Effects of hydration on the protonation state of a lysine analog crossing a phospholipid bilayer – insights from molecular dynamics and free-energy calculations†
Abstract
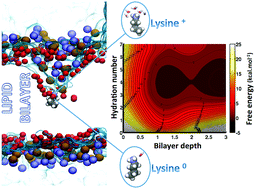
The low bioavailability of most therapeutic compounds is often counterbalanced by association with molecular vectors capable of crossing cell membranes. Previous studies demonstrated that for vectors bearing titratable chemical groups, the translocation process might be accompanied by a change in the protonation state. For simple compounds e.g. a lysine analog, free energy calculations, using a single collective variable, namely the insertion depth, suggest that such a transition could only take place if the amino acid diffuses deep enough into the hydrophobic core of the membrane, a situation thermodynamically unfavorable. Here, we determined the 2D potential of mean force associated with the translocation of lysine across a model membrane using as reaction coordinates not only its location in the bilayer but also its hydration. Our results cogently demonstrate that the change in protonation can result from a small fluctuation in the latter, even at low insertion depth.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...