Photodegradation of methyl thioglycolate particles as a proxy for organosulphur containing droplets†
Abstract
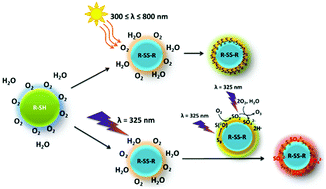
Understanding the formation and transformation of sulphur-rich particles is of prime importance since they contribute to the global atmospheric sulphur budget. In this work, we performed a series of experiments on a photoactive organosulphur compound namely, methyl thioglycolate, as a model of an organosulphur species of marine origin. By investigating the photoproducts within levitated droplets, we showed that elemental sulphur (α-S8) and sulphate (SO42−) can be photochemically generated at the gas–liquid interface by heterogeneous interaction with gaseous O2 and H2O. These results demonstrate that the surface of levitated droplets facilitate the oxidation of methyl thioglycolate in the dark, while illumination is necessary to produce the oxidation in bulk experiments.


 Please wait while we load your content...
Please wait while we load your content...