Self-assembly of chiral (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine into low-dimensional aluminophosphate materials driven by their amphiphilic nature†
Abstract
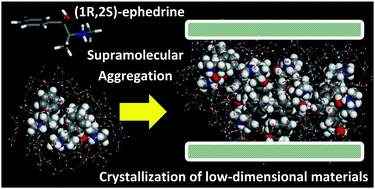
In an attempt to promote the crystallization of chiral inorganic frameworks, we explore the ability of chiral (1R,2S)-ephedrine and its diastereoisomer (1S,2S)-pseudoephedrine to act as organic building blocks for the crystallization of hybrid organo-inorganic aluminophosphate frameworks in the presence of fluoride. These molecules were selected because of their particular molecular asymmetric structure, which enables a rich supramolecular chemistry and a potential chiral recognition phenomenon during crystallization. Up to four new low-dimensional materials have been produced, wherein the organic molecules form an organic bilayer in-between the inorganic networks. We analyze by molecular simulations the trend of these chiral molecules to form these types of framework, which is directly related to their amphiphilic nature that triggers a strong self-assembly through hydrophobic interactions between aromatic rings and hydrophilic interactions with the fluoro-aluminophosphate inorganic units. Such a self-assembly process is strongly dependent on the concentration of the organic molecules.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...