Determination of graphene's edge energy using hexagonal graphene quantum dots and PM7 method†
Abstract
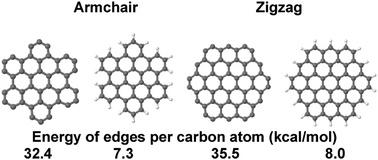
Graphene quantum dots (GQDs) are important for a variety of applications and designs, and the shapes of GQDs rely on the energy of their boundaries. Presently, many methods have been developed for the preparation of GQDs with the required boundaries, shapes and edge terminations. However, research on the properties of GQDs and their applications is limited due to the unavailability of these compounds in pure form. In the present computational study, the standard enthalpy of formation, the standard enthalpy of formation of edges and the standard enthalpy of hydrogenation are studied for hexagonal GQDs with purely zigzag and armchair edges in non-passivated and H-passivated forms using the semiempirical quantum chemistry method pm7. The standard enthalpy of formation of the edge is found to remain constant for GQDs studied in the range of 1 to 6 nm, and the enthalpies of edge C atoms are 32.4 and 35.5 kcal mol−1 for armchair and zigzag edges, respectively. In contrast to some literature data, the standard enthalpy of formation of hydrogenated edges is far from zero, and the values are 7.3 and 8.0 kcal mol−1 C for armchair and zigzag edges, respectively. The standard enthalpy of hydrogenation is found to be −10.2 and −9.72 eV nm−1 for the armchair and zigzag edges, respectively.


 Please wait while we load your content...
Please wait while we load your content...