Effect of amino group protonation on the carboxyl group in aqueous glycine observed by O 1s X-ray emission spectroscopy
Abstract
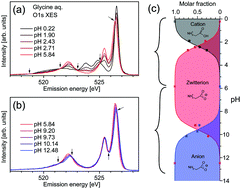
The valence electronic structures of the amino acid glycine in aqueous solution were investigated in detail through X-ray emission spectroscopy at O 1s excitation under selective excitation conditions of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O site in the carboxyl group. The X-ray emission spectra of glycine were similar to that of acetic acid (CH3COOH), suggesting a resemblance between the molecular orbitals associated with the carboxyl groups in the two molecules. The changes of O 1s X-ray emission spectra as a function of pH were investigated in detail. In addition to spectral changes due to protonation/deprotonation of the carboxyl group for lower pH-values around the pKa value (∼2.3), the spectra of glycine exhibited further changes in the higher-pH region near the pKb value of glycine (dissociation constant of amino group ∼9.5). These results show the effects of amino group protonation on the electronic state around the carboxyl group. X-ray emission spectroscopy might be a tool to investigate intramolecular interactions between functional groups in a molecule.
O site in the carboxyl group. The X-ray emission spectra of glycine were similar to that of acetic acid (CH3COOH), suggesting a resemblance between the molecular orbitals associated with the carboxyl groups in the two molecules. The changes of O 1s X-ray emission spectra as a function of pH were investigated in detail. In addition to spectral changes due to protonation/deprotonation of the carboxyl group for lower pH-values around the pKa value (∼2.3), the spectra of glycine exhibited further changes in the higher-pH region near the pKb value of glycine (dissociation constant of amino group ∼9.5). These results show the effects of amino group protonation on the electronic state around the carboxyl group. X-ray emission spectroscopy might be a tool to investigate intramolecular interactions between functional groups in a molecule.


 Please wait while we load your content...
Please wait while we load your content...