Combinatorial selection of a two-dimensional 3d-TM-tetracyanoquinodimethane (TM-TCNQ) monolayer as a high-activity nanocatalyst for CO oxidation†
Abstract
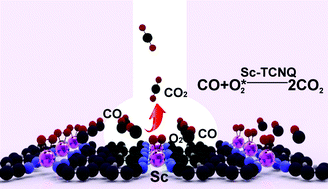
The CO oxidation reaction on single 3d-transition metal catalytic sites in experimentally realized tetracyanoquinodimethane (TM-TCNQ) monolayers (TM = Sc–Zn) is systematically investigated by means of first-principles calculations. Considering the stabilities, adsorption characteristics and thermodynamics of all the ten candidates (Sc–Zn), Sc-TCNQ is found to display the lowest activation energies and yield the highest catalytic activity for room temperature CO oxidation. Exploring the Langmuir–Hinshelwood (LH) and Eley–Rideal (ER) mechanisms, we find that the rate-limiting step of CO oxidation catalyzed by Sc-TCNQ (CO + O2* → OOCO*) can follow the LH mechanism with free energy barriers as low as 0.73 eV at 300 K. The second step of CO + O* → CO2 can occur with rather small energy barriers via either LH or ER mechanisms. The high activity of Sc-TCNQ can be attributed to its unique structural and electronic features by possessing high stability, optimum adsorption energies with adsorbates, and fast reaction kinetics. These results have significant implications for the synthesis of two-dimensional single atom catalysis for CO oxidation with low-cost and high activity at low temperature.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...