Site-specific electronic structure of imidazole and imidazolium in aqueous solutions†
Abstract
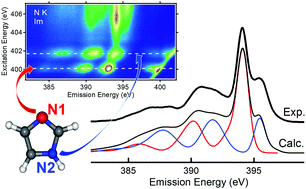
The occupied and unoccupied electronic structure of imidazole (C3N2H4) and imidazolium (C3N2H5+) in aqueous solutions is studied by X-ray emission spectroscopy (XES) and resonant inelastic soft X-ray scattering (RIXS). Both systems show distinct RIXS fingerprints with strong resonant effects. A comparison with calculated X-ray emission spectra of isolated imidazole and imidazolium suggests only a small influence of hydrogen bonding in the aqueous solution on the electronic structure of imidazole and imidazolium, and allows the attribution of specific spectral features to the non-equivalent nitrogen and carbon atoms in the molecules. In the case of nitrogen, this can also be achieved by site-selective resonant excitation. Furthermore, we find spectator shifts and symmetry selectivity in the RIXS spectra, as well as indications for rapid proton dynamics on the femtosecond timescale of the RIXS process, and derive the HOMO–LUMO gaps for the two molecules in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...