Influences of the molecular fuel structure on combustion reactions towards soot precursors in selected alkane and alkene flames†
Abstract
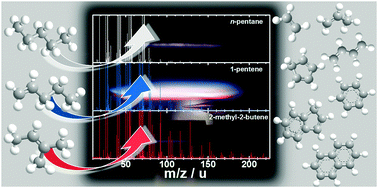
In this study, we experimentally investigate the high-temperature oxidation kinetics of n-pentane, 1-pentene and 2-methyl-2-butene (2M2B) in a combustion environment using flame-sampling molecular beam mass spectrometry. The selected C5 fuels are prototypes for linear and branched, saturated and unsaturated fuel components, featuring different C–C and C–H bond structures. It is shown that the formation tendency of species, such as polycyclic aromatic hydrocarbons (PAHs), yielded through mass growth reactions increases drastically in the sequence n-pentane < 1-pentene < 2M2B. This comparative study enables valuable insights into fuel-dependent reaction sequences of the gas-phase combustion mechanism that provide explanations for the observed difference in the PAH formation tendency. First, we investigate the fuel-structure-dependent formation of small hydrocarbon species that are yielded as intermediate species during the fuel decomposition, because these species are at the origin of the subsequent mass growth reaction pathways. Second, we review typical PAH formation reactions inspecting repetitive growth sequences in dependence of the molecular fuel structure. Third, we discuss how differences in the intermediate species pool influence the formation reactions of key aromatic ring species that are important for the PAH growth process underlying soot formation. As a main result it was found that for the fuels featuring a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, the chemistry of their allylic fuel radicals and their decomposition products strongly influences the combination reactions to the initially formed aromatic ring species and as a consequence, the PAH formation tendency.
C double bond, the chemistry of their allylic fuel radicals and their decomposition products strongly influences the combination reactions to the initially formed aromatic ring species and as a consequence, the PAH formation tendency.
- This article is part of the themed collection: Bunsentagung 2018: Kinetics in the Real World


 Please wait while we load your content...
Please wait while we load your content...