Molecular dynamics investigations of cello-oligosaccharide recognition by Cel9G–CBM3c from Clostridium cellulovorans†
Abstract
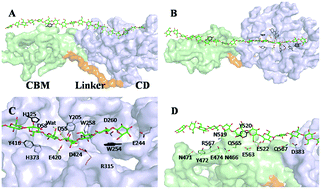
The processive mechanism of cellulases against cellulose represents one of the key mechanisms in the conversion of biomass. A reliable model of substrate binding in a multidomain cellulase is a prerequisite for fully understanding this mechanism. In this study, the specificity of the recognition of the polysaccharide by the multidomain endoglucanase Cel9G from Clostridium cellulovorans was investigated by molecular dynamics simulations. Aromatic ring-containing residues were found to be critical for stabilizing the substrate. The calculated subtotal contributions of polar residues close to the active site, e.g., D58, E244, R315 and D420, also have some critical functions in substrate binding. Unlike other members of the carbohydrate-binding module family, CBM3c alone is shown not to bind cellulose very well, which is also consistent with experimental conclusions.


 Please wait while we load your content...
Please wait while we load your content...