Spatial requirement for PAMO for transformation of non-native linear substrates†
Abstract
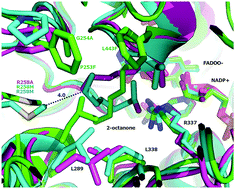
Phenylacetone monooxygenase is the most stable and thermo-tolerant member of the Baeyer–Villiger monooxygenases family, and therefore it is an ideal candidate for the synthesis of industrially relevant ester or lactone compounds. However, its limited substrate scope has largely limited its industrial applications. Linear substrates are interesting from an industrial point of view, it is thus necessary to identify the essential spatial requirement for achieving high conversions for non-native linear substrates. Here using molecular dynamics simulations, we compared the conversion of a non-native linear substrate 2-octanone and the native substrate phenylacetone, catalyzed by the WT enzyme and a quadruple variant P253F/G254A/R258M/L443F that exhibits significantly improved activity towards 2-octanone. We uncovered that a remarkable movement of L289 is crucial for a reshaping of the active site of the quadruple variant so as to prevent the aliphatic substrate from moving away from the C4a-peroxyflavin, thus enabling it to keep a catalytically relevant pose during the oxygenation process. By performing steady-state kinetic analysis of two single-mutation variants at position 258, we further validated that the L289 reposition is attributed to the combined effect of quadruple mutations. In order to further explore the substrate scope of PAMO we also studied the binding of cyclopentanone and 2-phenylcyclohexanone, which are the typical substrates of CPMO in group I and CHMO in group III, respectively. Our study provides fundamental atomic-level insights in rational engineering of PAMO for wide applications in industrial biocatalysis, in particular, in the biotransformation of long-chain aliphatic oils into potential biodiesels.


 Please wait while we load your content...
Please wait while we load your content...