Phase equilibrium and physical properties of biobased ionic liquid mixtures†
Abstract
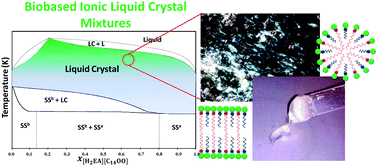
Protic ionic liquid crystals (PILCs) obtained from natural sources are promising compounds due to their peculiar properties and sustainable appeal. However, obtaining PILCs with higher thermal and mechanical stabilities for product and process design is in demand and studies on such approaches using this new IL generation are still scarce. In this context, this work discloses an alternative way for tuning the physicochemical properties of ILCs by mixing PILs. New binary mixtures of PILs derived from fatty acids and 2-hydroxy ethylamines have been synthesized here and investigated through the characterization of the solid–solid–[liquid crystal]–liquid thermodynamic equilibrium and their rheological and critical micellar concentration profiles. The mixtures presented a marked nonideal melting profile with the formation of solid solutions. This work revealed an improvement of the PILCs’ properties based on a significant increase in the ILC temperature domain and the obtainment of more stable mesophases at high temperatures when compared to pure PILs. In addition, mixtures of PILs also showed significant changes in their non-Newtonian and viscosity profile up to 100 s−1, as well as mechanical stability over a wide temperature range. The enhancement of the physicochemical properties of PILs here disclosed by such an approach leads to more new possibilities of their industrial application at high temperatures.


 Please wait while we load your content...
Please wait while we load your content...