The structure of a lanthanide complex at an extractant/water interface studied using heterodyne-detected vibrational sum frequency generation†
Abstract
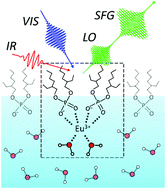
Solvent extraction plays an integral part in the separation and purification of metals. Because extractants generally used as complexing agents for metal extractions, such as di-(2-ethylhexyl)phosphoric acid (HDEHP) for lanthanide extractions, are amphiphilic, they come to an organic/water interface, and the interface plays a crucial role as the site of the formation of metal complexes and the subsequent transfer reaction to an organic phase. Despite the importance of the interface for metal solvent extractions, its molecular-level structure is unclear because of the experimental difficulties. Here we studied the structure of a trivalent europium (Eu3+) complex with HDEHP formed at the HDEHP monolayer/water interface using heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy. The study on the HDEHP/water interface enables us to investigate the structure of the interfacial Eu3+ complex by excluding the migration of Eu3+ into an organic phase after the complex formation at the interface. The interface-selective vibrational Imχ(2) spectra observed using HD-VSFG of the interface of HDEHP/aqueous Eu(NO3)3 solution in the 2800–3500 cm−1 region indicate that Eu3+ at the HDEHP/water interface is bonded by HDEHP from the air side and by water molecules from the water side. To the best of our knowledge, such metal complex structures have not been identified in organic or water solutions.


 Please wait while we load your content...
Please wait while we load your content...