Role of surface adsorption in tuning the properties of black phosphorus
Abstract
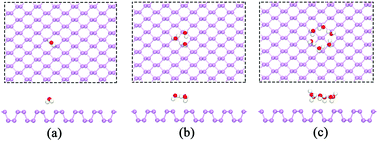
H2O and O2 are believed to be key factors that influence the structural stability of black phosphorus (BP) in ambient conditions. In this work, the interactions of H2O and/or O2 with BP are investigated using first-principles calculations. The results indicate that water molecules prefer to adsorb on the BP surface and form a six-member water ring. The dissociation barrier of O2 is significantly reduced in the presence of H2O, which dramatically promotes the degradation of BP. Moreover, the introduction of O2 also facilitates the adsorption of water on the surface. The effects of H2O and/or O2 on the quasiparticle band gap and exciton binding energy of BP are also investigated. The results suggest that water adsorption has only a slight influence on the electronic properties and exciton binding energy, while O2 adsorption causes obvious changes in the properties of BP, which results in a direct-to-indirect band gap transition in BP.


 Please wait while we load your content...
Please wait while we load your content...