Halo-phenyl based linear dipodal receptors for entrapment of anions/anionic associations within neutral non-cooperative self-assemblies†
Abstract
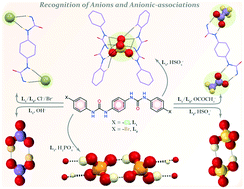
Two para-phenylenediamine based para-halophenyl substituted linear bis-urea receptors, L1 (para-chloro) and L2 (para-bromo), have been designed and synthesized to investigate their possible coordination with anions of various dimensions in the solid state. Both para-halophenyl-based isomers L1 and L2 form similar non-cooperative neutral self-assemblies with larger halides (i.e. chloride or bromide anions) and with planar acetate anions in solid state, although solution state studies indicate the binding tendency to be in the order of acetate > chloride > bromide, as evidenced from the 1H-NMR chemical shift values. In presence of excess strongly basic hydroxide anion, receptor L1 forms bicarbonate dimer (HCO3)2 entrapped neutral self-assemblies by OH− induced atmospheric CO2 fixation. Interestingly, in presence of tetrabutylammonium bisulfate anion, receptor L1 shows non-cooperative capture of divalent sulfate (SO42−) anion, whereas a bisulfate (HSO4)2 dimeric association has been entrapped within the non-cooperative self-assemblies of receptor L2. Furthermore, the para-halophenyl substituted isomer L1 also entraps linear (H2PO4)n polymeric aggregated anionic association within the neutral non-cooperative host-assemblies by hydrogen-bonding interactions.


 Please wait while we load your content...
Please wait while we load your content...