From light to heavy alkali metal tetraphosphonates (M = Li, Na, K, Rb, Cs): cation size-induced structural diversity and water-facilitated proton conductivity†
Abstract
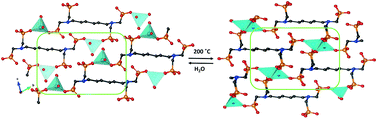
A family of alkali metal-based frameworks containing the tetraphosphonate ligand hexamethylenediamine-N,N,N′,N′-tetrakis(methylenephosphonic acid), HDTMP, is reported. A cation size-induced structural diversity, from monodimensional solids (Li+ and Na+) through layered (K+) to pillared-layered (Rb+ and Cs+) structures, was found. The proton conductivity properties of the Li compounds (hydrated and dehydrated) are reported and the influence of dehydration/rehydration processes in enhancing proton transfer processes is highlighted. Reversible changes in the dimensionality occurred upon full dehydration/rehydration with minor rearrangements in the framework, implying variations in the Li+–ligand connectivity but preserving the tetracoordination of the metal ion. The reversibly dehydrated–rehydrated sample displayed the highest proton conductivity (5 × 10−3 S cm−1 at 80 °C and 95% RH), a behavior attributed to reversible formation/reformation of P–O(H)–Li bonds that, in turn, provoked changes in the acidity of acid groups and water mobility in the temperature range of impedance measurements.


 Please wait while we load your content...
Please wait while we load your content...