Chemical reaction mechanism of ZnO grown using DEZn and N2O in MOCVD†
Abstract
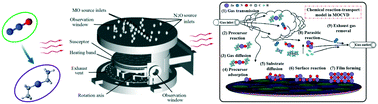
ZnO thin films were prepared by metal–organic chemical vapor deposition (MOCVD) using diethylzinc (DEZn) and N2O. The chemical reaction process of DEZn and N2O in the gas phase was studied by density functional theory and computational fluid dynamics calculation. Kinetic and thermodynamic data provide a better understanding of the ZnO deposition process. The deposition rate of ZnO in the reaction chamber was simulated by kinetic parameters, and the dependence of the film growth rate on temperature was demonstrated. The maximum ZnO film growth rate was at 973–1273 K when grown using DEZn and N2O in a high-speed rotating horizontal chamber. When the temperature exceeded 1273 K, the parasitic reaction led to a rapid decline in the ZnO deposition rate. In addition, multi-component migration and chemical reaction were analyzed, and the complete mechanism of DEZn and N2O in the reaction chamber was given.


 Please wait while we load your content...
Please wait while we load your content...