Switchable dielectric phase transition behaviors in two organic–inorganic copper(ii) halides with distinct coordination geometries†
Abstract
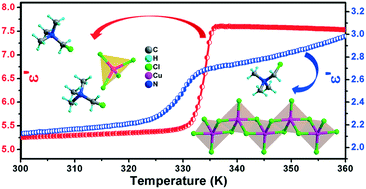
Two new organic–inorganic chlorocuprates(II), [(ClCH2)(CH3)3N]2CuCl4 (1), which contains a discrete [CuCl4]2− tetrahedron, and [(ClCH2)(CH3)3N]CuCl3 (2), which comprises one-dimensional [CuCl3]− chains of an edge-sharing CuCl5 pyramid, have been synthesized. Compound 1 exhibits a symmetry-breaking phase transition at 335.2 K, while 2 undergoes an isostructural phase transition at 333.8 K, as disclosed by differential scanning calorimetry measurements and variable-temperature X-ray diffraction analyses. The dielectric measurements reveal that both compounds 1 and 2 show prominent switchable dielectric activities between high and low dielectric states in the vicinity of the phase transition temperature. In addition, the contrast between the two dielectric states in 1 is much higher than that in 2, which is related to the more remarkable dynamic change of the [(ClCH2)(CH3)3N]+ cation in 1 during the phase transition process. It is believed that this work will pave a new avenue for the design of multifunctional phase transition materials by exploring the area of Cu2+-based organic–inorganic metal halides.


 Please wait while we load your content...
Please wait while we load your content...