Dicarboxylate-induced structural diversity of luminescent ZnII/CdII coordination polymers derived from V-shaped bis-benzimidazole†
Abstract
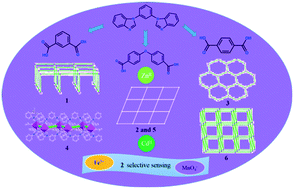
Six new coordination polymers (CPs) based on the V-shaped 1,3-bis(benzimidazolyl)benzene (bbib) have been synthesized under solvothermal conditions: [Zn(bbib)(ip)]n (1), {[Zn(bbib)(oba)]·solvents}n (2), {[Zn(bbib)(tp)]·DMF}n (3), [Cd(bbib)(ip)]n (4), {[Cd(bbib)(oba)]·H2O·solvents}n (5) and {[Cd(bbib)(tp)]·DMF}n (6) (ip = isophthalate, oba = 4,4′-oxybisbenzoate and tp = terephthalate). Complexes 1–3 display a plane minimal net, (4,4) sheet and a hexagonal plane net, respectively. The structures of 4–6 range from a 1D zigzag chain (4), 2D (4,4) sheet (5) to a 3D “pillar-layer” coordination framework with pcu α-Po topology (6). The structural transition from 1D to 3D among these complexes is realized with the aid of different auxiliary ligands. The results demonstrate that the conformations and coordination modes of dicarboxylate ligands make an impact on the structural diversity of ZnII/CdII coordination polymers. To the best of our knowledge, the CPs based on the bbib ligand have been successfully prepared for the first time. Moreover, complex 2 is a good bifunctional chemosensor for the detection of both Fe3+ and MnO4− with the detection limits of 3.31 ppm and 8.81 ppm, respectively, indicating that 2 could be used as an efficient fluorescent sensor with high sensitivity and selectivity for Fe3+ and MnO4−.


 Please wait while we load your content...
Please wait while we load your content...