Structures and phase transitions in molecular complexes containing tetrafluoroboric acid and tetramethylpyrazine†
Abstract
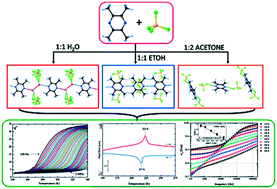
Crystals of tetrafluoroboric acid with tetramethylpyrazine, TMP + HBF4 + H2O (1 : 1 : 2, A), TMP + HBF4 (1 : 1, B) and TMP + HBF4 (1 : 2, C) were synthesized and characterized by physicochemical experimental methods. The crystal structures for A and B were determined at 100 K. Since C undergoes a phase transition at 217/223 K (cooling/heating) the structures were measured at 100 and 250 K. Infinite chains of molecules linked via the hydrogen bonds are present in the crystals A and B. A BF4−⋯TMPH22+⋯BF4− assembly was found in the crystal structure of C. The dielectric responses have been measured for all three crystals, A, B and C, in a wide temperature (160–300 K) and frequency range (100 Hz–2 MHz). The mechanism of the phase transition at 217/223 K found in C as well as the origin of the ac conductivity was determined to be related to the dynamics of protons in the hydrogen bonds.


 Please wait while we load your content...
Please wait while we load your content...