Kinetics during hydrothermal synthesis of nanosized KxNa1−xNbO3†
Abstract
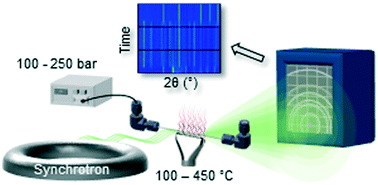
Hydrothermal synthesis is an efficient method of making nanosized functional oxide materials, and it has also been used to synthesize KxNa1−xNbO3 solid solution, a promising lead-free piezoelectric material. However, the control of nucleation and growth during synthesis to tailor the properties of materials is challenging and this work aimed to solve the challenges reported regarding the stoichiometry and size of KxNa1−xNbO3 formed by hydrothermal synthesis by in situ X-ray diffraction. KxNa1−xNbO3 crystallites of 18–65 nm with a controlled stoichiometry were produced by varying the Na/K ratio in the precursor solution, the solvent type and the final reaction temperature. The reaction proceeds via rapid formation of a potassium sodium hexaniobate intermediate phase during heating, which converts to KxNa1−xNbO3 at the reaction temperature. The Na occupancy in KxNa1−xNbO3 was found to correlate with the Na content in the intermediate phase and decreases by using a mix of ethanol and water as a solvent instead of only water. We show that in situ techniques are powerful for providing insight into the determining factors when preparing oxide nanoparticles with a designed composition, structure and size.


 Please wait while we load your content...
Please wait while we load your content...