Charge control of the formation of two neutral/cationic metal–organic frameworks based on neutral/cationic triangular clusters and isonicotinic acid: structure, gas adsorption and magnetism†
Abstract
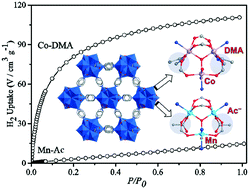
Two neutral/cationic metal–organic frameworks (MOFs), [Mn3(μ3-OH)(in)4(CH3COO)(H2O)]·H2O (Mn-Ac) and [Co3(μ3-OH)(in)4(DMA)2(H2O)]ClO4·DMA·3H2O (Co-DMA), based on triangular secondary building units (SBUs) MII3(OH)(O2CR)4L3 (M = Mn/Co, R = linker group, and L = coligand) with the same hex topology are successfully constructed using an isonicotinic acid (Hin) ligand, and their framework charges are controlled by the synergistic effects of counterions, solvent and metal centers. Three MnII/CoII ions were blocked by carboxylates from Hin and μ3-OH, forming triangular cationic [MII3(OH)(O2C)4]+ units. Then in Mn-Ac, the CH3COO− counterion of the manganese(II) salt is used as a coligand, and it could be preemptively coordinated with the metal to obtain the neutral SBU. In addition, Mn-Ac possesses a neutral framework with low symmetry and trapezium-shaped channels with small effective pore sizes. When using Co(ClO4)2 instead of Mn(CH3COO)2 in compound Co-DMA, the solvent DMA molecules proactively blocked the remaining cobalt(II) sites of [CoII3(OH)(O2C)4]+, forming cationic SBUs. Therefore, the cationic framework Co-DMA was obtained, which shows open square-shaped channels. The desolvated framework of Co-DMA displays a Langmuir surface area of 839 m2 g−1 and more effective sorption capacities for N2, CO2 and H2 than those of Mn-Ac, which is probably because the cationic Co-DMA has a larger effective aperture and stability than the neutral Mn-Ac. Magnetic studies show dominant antiferromagnetic behaviours for both Mn-Ac and Co-DMA.


 Please wait while we load your content...
Please wait while we load your content...