Exploring the syntheses, structures, topologies, luminescence sensing and magnetism of Zn(ii) and Mn(ii) coordination polymers based on a semirigid tricarboxylate ligand†
Abstract
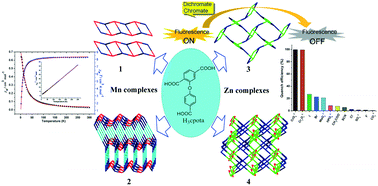
Solvothermal reactions of Zn(II) or Mn(II) salts and 2-(4-carboxyphenoxy)terephthalic acid (H3cpota) in the presence of N-heterocyclic group ligands (1,10-phenanthroline (phen) or 1,4-di(1H-imidazol-1-yl)benzene (bib) or 4,4′-bipyridine (4,4′-bpy)) produced four multi-dimensional coordination polymers, namely, [Mn(μ3-Hcpota)(phen)]n·nH2O 1 (1D), [Mn2(μ4-Hcpota)(μ3-Hcpota)(μ2-bib)2(H2O)]n2 (3D), [Zn(μ3-Hcpota)(phen)]n·nH2O 3 (2D), and [Zn2(μ4-cpota)(μ2-4,4′-bpy)(μ3-OH)]n·nH2O 4 (3D). Complex 1 features a one-dimensional double ladder formed by syn–anti carboxylate-bridged dimeric Mn(II) units with 8- and 26-membered rings in an alternating pattern sharing three atoms and gives a (42·6) topology. Complex 2 is formed by linear inorganic chains sharing Mn(II) 8-membered rings in double syn–syn carboxylate bridges and H-bond R11(8) alternate modes, which are further connected to each other by two symmetry independent Hcpota2− ligands as well as coordinated bib bridges giving rise to a three-dimensional net with a novel (4·62)(4·68·8)(43·611·8)(43·63) topology. Complex 3 exhibits a two-dimensional layered structure containing 36-membered rings based on 8-membered dinuclear Zn(II) units with (32·44)(34·46·64·7) fes-net topology. The phen and benzoic acid groups are like two kinds of blades in the two sides of the two-dimensional structure of 3. Complex 4 is an interesting three-dimensional (3,8)-connected network including tetranuclear tricyclic [Zn4(OH)2(COO)6] clusters with a new (4·5·6)2(42·56·616·72·82) topology. The semirigid tricarboxylate ligand (H3cpota) shows three geometric modes in the self-assembly of four coordination polymers: a) ‘L’ shape in 1, b) ‘I’ shape in 2 and 3, and c) ‘T’ shape in 4. Magnetization analyses disclose the antiferromagnetic behaviors of 1 and 2. Complexes 3 and 4 feature good solvent stability and can withstand ten common solvents. Luminescence investigations reveal that complex 3 has a highly sensitive, selective, low detection limit and well-recyclable properties in the detection of hexavalent chromium anions in aqueous solution. Quenching mechanisms are also studied in detail.


 Please wait while we load your content...
Please wait while we load your content...