Construction of metal–organic frameworks (MOFs) and highly luminescent Eu(iii)-MOF for the detection of inorganic ions and antibiotics in aqueous medium†
Abstract
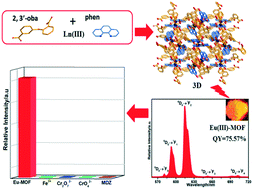
The hydrothermal reaction of Ln(NO3)3·6H2O with 2,3′-oxybis(benzoic acid) (2,3′-H2oba) and 1,10-phenanthroline (phen) afforded the metal–organic frameworks (Ln-MOFs), [Ln2(2,3′-oba)3(phen)2]n (Ln = La 1, Eu 2, Gd 3, and Tb 4). 1 possesses a 3D network with [La1O7N2] and [La2O7N2] polyhedra, and 2–4 exhibit similar 3D networks containing [Ln1O7N2] and [Ln2O6N2] polyhedra. These complexes are thermally stable in air and have high stability in water in a wide pH range (acidic pH = 3 and alkaline pH = 13 solutions). Notably, MOFs 2 and 4 display bright red and green emission with the high fluorescence quantum yields of 75.57% and 41.86%, respectively. The highly luminescent chemically stable Eu-MOF shows multi-responsive luminescence sensing functions to detect Fe3+, Cr6+, and metronidazole (MDZ) in aqueous medium even in acidic conditions (pH = 3) via a fluorescence quenching mechanism. Excess Fe3+, Cr6+, and antibiotics can cause environmental pollution and serious diseases; thus, their detection is very important. In addition, one of the important advantages of Eu-MOF is that this complex can be excited at a long excitation wavelength (352 nm), which is in the near-visible radiation region of 350 nm < λex < 420 nm, and is an important requirement for its applications.


 Please wait while we load your content...
Please wait while we load your content...