Nitramino-functionalized tetracyclic oxadiazoles as energetic materials with high performance and high stability: crystal structures and energetic properties†
Abstract
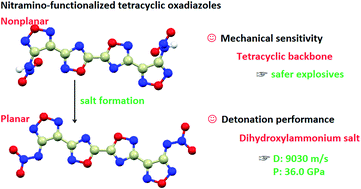
A series of tetracyclic energetic materials were developed through the introduction of 1,2,4-oxadiazole into nitramino-1,2,5-oxadiazole. All these thirteen new compounds were fully characterized and seven of them were further confirmed by single crystal X-ray diffraction. Salt formation led to planarization of the parent structure, reduction in lengths, increase in bond-dissociation energies of N–NO2 bonds, and formation of hydrogen bonds, which significantly increased decomposition temperatures from 84 °C (the neutral compound) to 187–303 °C (the energetic salts). Thus, the increase in thermal stability falls in the range of 103–219 °C. To the best of our knowledge, 219 °C is the largest reported increase in thermal stability due to salt formation. In addition, electrostatic surface potentials and noncovalent interactions (π–π stacking) were analyzed to understand structure–property relationships of these compounds. Both theoretical calculations and practical explosive experiments indicate that the dihydroxylammonium salt exhibits better detonation performance than the powerful explosive RDX. The tetracyclic backbone provides these compounds with enhanced stability. Short synthesis steps, low cost, good thermostability, excellent detonation performance, and acceptable mechanical sensitivity highlight the practical applications of these energetic compounds.


 Please wait while we load your content...
Please wait while we load your content...