A basis for the kinetic selection of polymorphs during solution crystallization of organic compounds
Abstract
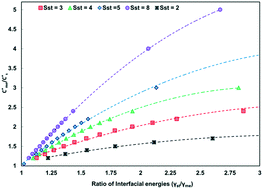
The equations of the classical nucleation theory were used to calculate the critical nucleation free energy for pairs of polymorphs. Conditions explored include supersaturation, the ratio of interfacial energies (γst/γme) and equilibrium solubility (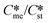 ) for pairs of polymorphs, crystallization temperature and molecular volume. Analysis of the critical free energies of nucleation of polymorphs pairs leads to an understanding of why we rarely see differences in excess of 2-fold in equilibrium solubilities for pairs of polymorphs. When this threshold is exceeded for low values of supersaturation with respect to the stable polymorph (Sst) the corresponding supersaturation with respect to the metastable polymorph (Sme) becomes very small, driving up the value of critical nucleation free energy for that polymorph. Essentially the model explains the change in relative kinetic accessibility of the polymorphs because for high values of
) for pairs of polymorphs, crystallization temperature and molecular volume. Analysis of the critical free energies of nucleation of polymorphs pairs leads to an understanding of why we rarely see differences in excess of 2-fold in equilibrium solubilities for pairs of polymorphs. When this threshold is exceeded for low values of supersaturation with respect to the stable polymorph (Sst) the corresponding supersaturation with respect to the metastable polymorph (Sme) becomes very small, driving up the value of critical nucleation free energy for that polymorph. Essentially the model explains the change in relative kinetic accessibility of the polymorphs because for high values of 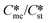 or low values of Sst the corresponding Sme value can become very small, driving up the value of the critical nucleation free energy of the metastable polymorph. A domain diagram has been developed by identifying for any selected values of supersaturation the combinations of
or low values of Sst the corresponding Sme value can become very small, driving up the value of the critical nucleation free energy of the metastable polymorph. A domain diagram has been developed by identifying for any selected values of supersaturation the combinations of 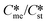 and γst/γme at which the critical nucleation free energy of the polymorphs are equal. This diagram can be used to select experimental conditions; most notably the supersaturation needed for a given polymorph pair to select kinetically the metastable or stable forms.
and γst/γme at which the critical nucleation free energy of the polymorphs are equal. This diagram can be used to select experimental conditions; most notably the supersaturation needed for a given polymorph pair to select kinetically the metastable or stable forms.


 Please wait while we load your content...
Please wait while we load your content...