Pasteur's tartaramide/malamide quasiracemates: new entries and departures from near inversion symmetry†
Abstract
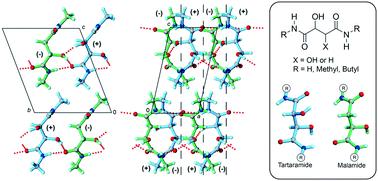
This investigation explores Pasteur's 1853 (+)-tartaramide/(−)-malamide quasiracemate, extends this system to the crystallization behavior of methyl and butyl derivatives, and challenges the well-known notion that pairs of quasienantiomers assemble without exception to give near centrosymmetric crystal packing motifs. Each of the structures included in this study exhibits an extensive set of N/O–H⋯O contacts with component alignment in the quasiracemates defined by inversion and/or glide symmetry. Solid-state density functional theory calculations were applied to quantify the crystal stabilization and rationalize the observed packing tendencies.


 Please wait while we load your content...
Please wait while we load your content...
