A multi-component mass transfer rate based model for simulation of non-equilibrium growth of crystals
Abstract
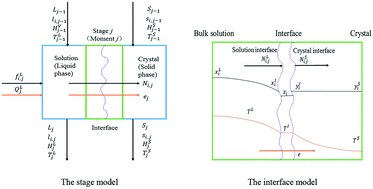
A model based on multi-component mass transfer is proposed for modeling the non-equilibrium growth behavior of crystals during solution crystallization. The multi-component composition in crystals in any spatial location can thus be estimated at any time during a crystallization process. It can be applied to the estimation of impurity content and assessing the stability of crystalline pharmaceuticals. The multi-components are equally described by diffusion, adsorption and integration equations. The facet growth rates are estimated by the amount of materials grown on the surface divided by the material densities and the surface areas. This is unlike the conventional facet growth kinetic model in which the growth rate is correlated directly to supersaturation. The modeling method is illustrated by case studies of NaNO3 and KDP crystallization. The dynamic evolution of crystal composition and shape distribution is simulated.


 Please wait while we load your content...
Please wait while we load your content...