Pharmaceutical cocrystals of naringenin with improved dissolution performance†
Abstract
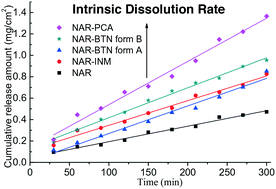
Naringenin (NAR), a natural flavanone compound, has received increasing attention in recent years due to its diverse pharmacological activities. However, this bioflavonoid shows poor solubility and therefore is difficult to absorb on oral ingestion. To improve the solubility of NAR, co-crystallization trials were performed, and four NAR cocrystals were obtained for the first time. All products were comprehensively characterized by a range of analytical methods, including single crystal and powder X-ray diffraction (XRD), liquid and solid state nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR), and differential scanning calorimetry (DSC). The dissolution studies revealed that all the four cocrystals, namely, NAR–isonicotinamide (NAR–INM), NAR–picolinic acid (NAR–PCA), and two modifications of NAR–betaine (NAR–BTN), exhibit improved apparent solubilities and intrinsic dissolution rates (IDRs) relative to the parent NAR. Two NAR–BTN forms are monotropically related. Additionally, BTN was used for the first time to form cocrystals with flavonoid compounds, which may provide crystallographic inspiration for the cocrystal design and screening of these kinds of bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...