On mechanisms of mesocrystal formation: magnesium ions and water environments regulate the crystallization of amorphous minerals†
Abstract
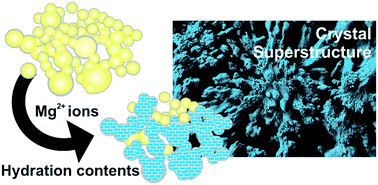
Nature produces hierarchical, functional materials by shaping amorphous mineral precursors under physiological conditions. Although biominerals inspire the architectures of synthetic counterparts, the biogenic phase transformations yielding precise crystalline forms, polymorphs and structures are unclear. Elucidating the transformation and structuration of amorphous minerals, herein we show distinct crystallization and structuration schemes synergistically controlled by environmental water contents and the Mg/Ca atomic ratio within amorphous mixed metal carbonates. Control of phase transformation, as well as resultant crystalline micro- and nano-structures, reflects the significance of the amorphous precursors of biominerals as disordered by design. Thereby, we complement the literature-known, suggested (bio)polymer-mediated ‘divide and protect’ mechanism of amorphous mineral stabilization by a Mg2+-based ‘unite and protect’ strategy. Altogether, this allows delineating a novel mechanism for mesocrystal formation based on the interface-coupled dissolution–re-precipitation of mesoscale amorphous precursors, which appear important in biomineralization. In the latter case, the recruitment of environmentally abundant Mg2+ species can also supplement the functions of biomolecules.


 Please wait while we load your content...
Please wait while we load your content...