Quantitative analysis of weak non-covalent interactions in (Z)-3-(4-halophenyl)-2-(pyridin-2/3/4-yl)acrylonitriles†
Abstract
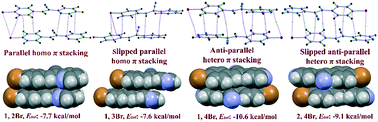
A detailed experimental and theoretical investigation on the intermolecular interactions in (Z)-3-(4-halophenyl)-2-(pyridin-2/3/4-yl)acrylonitriles is reported. The intermolecular interactions are analyzed and quantified by PIXEL, DFT and quantum theory of atoms in molecules (QTAIM) calculations. The results showed that the absence of strong classical hydrogen bonds and the presence of several weak intermolecular C–H⋯N, C–H⋯halogen, halogen⋯halogen and π stacking interactions play important roles in the stabilization of crystal structures. Four different π stacking dimers (homo parallel, homo slipped parallel, hetero anti-parallel and hetero anti-parallel slipped) are observed and found to be the most stabilized dimeric motifs in all structures. The hetero anti-parallel (−10.6 kcal mol−1) and slipped anti-parallel (−9.1 kcal mol−1) π⋯π interactions have the highest stabilization energies among the other interactions observed in the present study. The substituted halogen atoms (X = F, Cl and Br) are involved in the variant and invariant C–H⋯X, C⋯X and halogen⋯halogen interactions in all three families. The relative contributions of various non-covalent interactions are calculated using Hirshfeld surface analysis and 2D fingerprint plots. Further, detailed UV-vis spectral studies are reported for the title compounds in solution and solid phases. These results are compared with the simulated spectra and good agreement is found between experimental and theoretical spectra.


 Please wait while we load your content...
Please wait while we load your content...