Diversity of crystal structures and physicochemical properties of ciprofloxacin and norfloxacin salts with fumaric acid†
Abstract
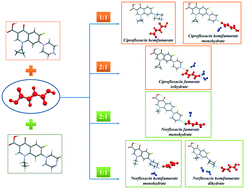
The crystallization of norfloxacin and ciprofloxacin – antibacterial fluoroquinolone compounds – with fumaric acid resulted in the isolation of six distinct solid forms of the drugs with different stoichiometries and hydration levels. Each salt can be selectively obtained by mechanochemical treatment in the presence of water/organic mixtures of a particular composition. The new phases were analysed using TG, DSC and PXRD, and their structural parameters were determined using single crystal X-ray diffraction. Despite having the same counterion, the ciprofloxacin and norfloxacin fumarates crystallise to form distinct crystal structures, which consequently determine the differences in the relative stability and the corresponding physicochemical properties of the solid forms. The influence of water activity (aw) on the solid form stability and transformation pathways of anhydrous and hydrated fumarates was elucidated. The solubility and phase stability of the salts were also investigated in pharmaceutically relevant buffer solutions with pH 6.8 and pH 1.2. The largest solubility improvement relative to the parent drug (≈33 times) in the pH 6.8 medium was observed in the case of ciprofloxacin hemifumarate sesquihydrate. In turn, the norfloxacin fumarates showed a moderate 3-fold enhancement in solubility.


 Please wait while we load your content...
Please wait while we load your content...