Nickel-catalysed decarbonylative borylation of aroyl fluorides†
Abstract
The first Ni(cod)2/PPh3 catalyst system has been established for decarbonylative borylation of aroyl fluorides with bis(pinacolato)diboron. A wide range of functional groups in the substrates were well tolerated. The ease of access of the starting aroyl fluorides indicates that these results might become an alternative to the existing decarbonylation events.
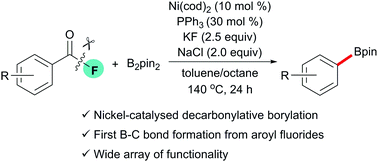


 Please wait while we load your content...
Please wait while we load your content...