Facile synthesis of unnatural β-germyl-α-amino amides via Pd(ii)-catalyzed primary and secondary C(sp3)–H bond germylation†
Abstract
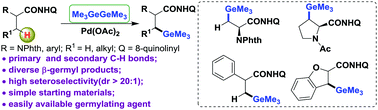
Pd(II)-Catalyzed direct C(sp3)–H germylation of α-AA derivatives with the assistance of a bidentate auxiliary for the efficient synthesis of β-germyl-α-amino amides is reported. This protocol features good generality for primary and secondary C–H bonds of aliphatic amides. Mechanistic studies show that a crucial five-membered palladacycle intermediate may play a key role in this process.


 Please wait while we load your content...
Please wait while we load your content...