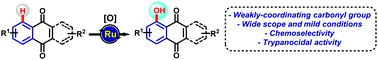
Ruthenium-catalyzed C–H oxygenation of quinones by weak O-coordination for potent trypanocidal agents†
Abstract
Ruthenium-catalysis enabled the C-5 selective C–H oxygenation of naphthoquinones, and also sets the stage for the site-selective introduction of a hydroxyl group into anthraquinones. A-ring modified naphthoquinoidal compounds represent an important class of bioactive quinones for which the present study encompasses the first C–H oxygenation strategy by weak O-coordination.


 Please wait while we load your content...
Please wait while we load your content...