Hydrophilic azaspiroalkenes as robust bioorthogonal reporters†
Abstract
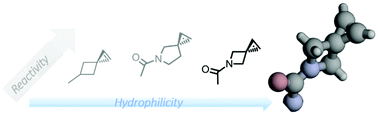
Two hydrophilic spiroalkenes, azaspiro[2.3]hex-1-ene and azaspiro[2.4]hept-1-ene, were designed and synthesized. Compared to the previously reported spiro[2.3]hex-1-ene, the azaspiroalkenes exhibited greater water solubility and reactivity as dipolarophiles in the photoinduced tetrazole–alkene cycloaddition reaction. In addition, an azaspiro[2.3]hex-1-ene-containing amino acid, AsphK, was found to be charged by an engineered pyrrolysyl-tRNA synthetase into proteins via amber codon suppression in E. coli as well as in mammalian cells.


 Please wait while we load your content...
Please wait while we load your content...