Construction of a diverse set of terpenoid decalin subunits from a common enantiomerically pure scaffold obtained by a biomimetic cationic cyclization†
Abstract
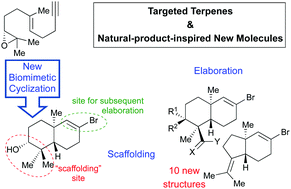
An unprecedented biomimetic cationic cyclization reaction of an alkyne-containing geraniol-derived epoxide is used for the stereoselective synthesis of a novel enantiomerically pure scaffold that is easily transformed into a set of structurally diverse decalin derivatives with potential application in the synthesis of targeted natural products and/or natural-product-inspired new molecules.


 Please wait while we load your content...
Please wait while we load your content...