A stereoselective sequential organocascade and multicomponent approach for the preparation of tetrahydropyridines and chimeric derivatives†
Abstract
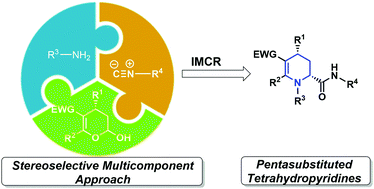
A stereoselective multicomponent approach leading to a novel class of pentasubstituted tetrahydropyridines is described. Variation of the components enabled the incorporation of peptide, sugar and steroid moieties to access chimeric derivatives. DFT calculations provide insights about the unprecedented high diastereoselectivity of the MCR.


 Please wait while we load your content...
Please wait while we load your content...