Tandem one-pot CO2 reduction by PMHS and silyloxycarbonylation of aryl/vinyl halides to access carboxylic acids†
Abstract
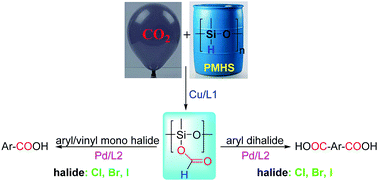
The present study discloses the synthesis of aryl/vinyl carboxylic acids from Csp2-bound halides (Cl, Br, I) in a carbonylative path by using silyl formate (from CO2 and hydrosilane) as an instant CO-surrogate. Hydrosilane provides hydride for reduction and its oxidation product silanol serves as a coupling partner. Mono-, di-, and tri-carboxylic acids were obtained from the corresponding aryl/vinyl halides.


 Please wait while we load your content...
Please wait while we load your content...